Using manure and compost as nutrient sources for fruit and vegetable crops
Manure is a valuable fertilizer for any farming operation and has been used for centuries to supply needed nutrients for crop growth. The use of manure has generally declined on many farms over the past 50 years due to: 1) Farm specialization with increasing separation of crop and livestock production, 2) Cost of transporting manure, which is a bulky, relatively low analysis nutrient source, and 3) Increased availability of high analysis synthetic fertilizers that usually provide a cheaper source per unit of nutrient than manure. Despite these limitations, manure (and other organic nutrient sources) produced on or near a vegetable farm provide many benefits and should be beneficially utilized whenever possible.
Manure and compost not only supply many nutrients for crop production, including micronutrients, but they are also valuable sources of organic matter. Increasing soil organic matter improves soil structure or tilth, increases the water-holding capacity of coarse-textured sandy soils, improves drainage in fine-textured clay soils, provides a source of slow release nutrients, reduces wind and water erosion, and promotes growth of earthworms and other beneficial soil organisms. Most vegetable crops return small amounts of crop residue to the soil, so manure, compost, and other organic amendments help maintain soil organic matter levels.
Proper use of manure and compost is essential from both a production and environmental standpoint. Applying rates that are too low can lead to nutrient deficiency and low yields. On the other hand, too high a rate can lead to nitrate leaching, phosphorus runoff, accelerated eutrophication of lakes, and excessive vegetative growth of some crops. Thus, understanding how to manage manure is important for any farming operation with livestock that relies on manure as a major source of nutrients, as well as for vegetable producers who have access to an economical supply of manure, compost, or other organic nutrient sources.
This discussion addresses differences between the composition of fresh and composted manure, nutrient availability from manure/compost, and calculation of how much manure/compost to apply. Although focused on manure or composted manure, much of the discussion and the methods for calculating rates are generally applicable to effective use of different types of compost, biosolids, and similar organic nutrient sources.
Nutrient Composition of Manure and Compost
Many different types of manure are available for crop production. For this discussion, it is assumed that most vegetable growers will be using solid manure with or without bedding. Similar principles will apply to the use of liquid manures. The nutrient content of manures varies with animal, bedding, storage, and processing. The approximate nutrient composition of various solid manures, including some composted manures, is presented in Table 1. While this table provides a general analysis of manure or compost nutrient content, it is strongly recommended that if routine applications are made for crop production the specific manure being used should be tested by a laboratory for moisture and nutrient content. Nutrient analysis should include: total nitrogen (N), ammonium-N, phosphate (P2O5), and potash (K2O). Accurate manure or compost analysis requires that a representative sample be submitted; so several subsamples should be collected and composited to make up the sample. If manure or compost is being purchased, request a nutrient analysis from the seller for N, P2O5, and K2O content.
Fresh vs. composted manure. Fresh, non-composted manure will generally have a higher N content than composted manure (Table 1). However, the use of composted manure will contribute more to the organic matter content of the soil. Fresh manure is high in soluble forms of N, which can lead to salt build-up and leaching losses if over applied. Fresh manure may contain high amounts of viable weed seeds, which can lead to weed problems. In addition, various pathogens such as E. coli may be present in fresh manure and can cause illness to individuals eating fresh produce unless proper precautions are taken. Apply and incorporate raw manure in fields where crops are intended for human consumption at least three months before the crop will be harvested. Allow four months between application and harvest of root and leaf crops that come in contact with the soil. Do not surface apply raw manure under orchard trees where fallen fruit will be harvested.
Heat generated during the composting process will kill most weed seeds and pathogens, provided temperatures are maintained at or above 131°F for 15 days or more (and the compost is turned so that all material is exposed to this temperature for a minimum of 3 days). The microbially mediated composting process will lower the amount of soluble N forms by stabilizing the N in larger organic, humus-like compounds. A disadvantage of composting is that some of the ammonia-N will be lost as a gas. Compost alone also may not be able to supply adequate available nutrients, particularly N, during rapid growth phases of crops with high nutrient demands. Composted manure is usually more expensive than fresh or partially aged manure.
Heat-dried manure/compost. Drying manure or compost to low moisture content reduces their volume and weight, which lowers transportation costs, but it also requires energy inputs. Dried products can be easier to handle and apply uniformly to fields, especially those that have been processed into pellets. Heat drying also reduces pathogens if temperatures exceed 150 to 175°F for at least one hour and water content is reduced to 10 to 12% or less. The significant energy costs to heat-dry manure or compost at high temperatures are in contrast to the self-heating generated by microbial respiration during the composting process. Heat-dried composts vary widely in the degree to which they are composted before drying. Many are only partially composted and have higher amounts of soluble (inorganic) N forms than mature, stable compost. This readily available N gives these products some characteristics that are similar to soluble N fertilizers, such as ammonium nitrate. Heat drying of manure and immature compost may increase volatilization of ammonia-N and reduce the total N content of the finished product. In addition, composted or partially composted material that is dried at high temperature rather than going through a curing phase at ambient temperatures is not as biologically active as mature compost. The disease suppressive properties of some composts depends upon recolonization of the compost by disease suppressing organisms during the curing phase.
Nutrient Availability from Manure and Compost
The analysis of manure or compost provides total nutrient content, but availability of the nutrients for plant growth will depend on their breakdown and release from the organic components. Generally, 70 to 80% of the phosphorus (P) and 80 to 90% of the potassium (K) will be available from manure the first year after application. Numbers from a table or from an analysis report should to be multiplied by these factors to obtain the amount of P2O5 and K2O available to crops from a manure or compost application.
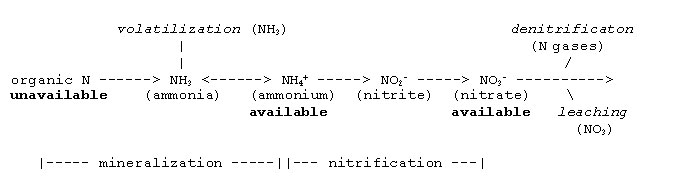
Calculating N availability is more complex than determining P and K availability. Most of the N in manure is in the organic form and essentially all of the N in compost is organic. Organic N is unavailable for uptake until microorganisms degrade the organic compounds that contain it. A smaller fraction of the N in manure is in the ammonium/ammonia or inorganic form. The ammonium-N form is a readily available fraction. Other inorganic forms such as nitrate and nitrite can also exist, but their quantities are usually very low. Estimated levels of ammonium-N and total N in fresh and composted manure are shown in Table 1.
When applied to soil, manure, compost, and other organic amendments undergo microbial transformations that release plant-available N over time. Volatilization, denitrification, and leaching result in N losses from the soil that reduce the amount of N that can be used by crops.
(all values are on a fresh weight basis).
|
Manure Type |
Dry Matter |
Ammonium-N |
Total N |
P2O5 |
K2O |
|
% |
————————- lb/ton ————————— |
||||
Swine, no bedding |
18 |
6 |
10 |
9 |
8 |
| Swine, with bedding |
18 |
5 |
6 |
7 |
7 |
| Beef, no bedding |
52 |
7 |
21 |
14 |
23 |
| Beef, with bedding |
50 |
8 |
21 |
18 |
26 |
| Dairy, no bedding |
18 |
4 |
9 |
4 |
10 |
| Dairy, with bedding |
21 |
5 |
9 |
4 |
10 |
| Sheep, no bedding |
28 |
5 |
18 |
11 |
26 |
| Sheep, with bedding |
28 |
5 |
14 |
9 |
25 |
| Poultry, no litter |
45 |
26 |
33 |
48 |
34 |
| Poultry, with litter |
75 |
36 |
56 |
45 |
34 |
| Turkey, no litter |
22 |
17 |
27 |
20 |
17 |
| Turkey, with litter |
29 |
13 |
20 |
16 |
13 |
| Horse, with bedding |
46 |
4 |
14 |
4 |
14 |
| Poultry compost |
45 |
1 |
17 |
39 |
23 |
| Dairy compost |
45 |
<1 |
12 |
12 |
26 |
| Mixed compost: Dairy/Swine/Poultry |
43 |
<1 |
11 |
11 |
10 |
aTotal N = Ammonium-N plus organic N
Sources: Livestock Waste Facilities Handbook, 2nd ed., 1985, Midwest Plan Service; Organic Soil Amendments and Fertilizers, 1992, Univ. of Calif. #21505.
Table 2 provides estimates of N availability from manure the first growing season after application. The actual amount available is dependent on manure type, bedding, and whether the manure has been composted. Usually 25 to 50% of the organic-N in fresh manure is available the first year. In addition to the organic fraction, N availability from manure also has to take into account the amount of ammonium-N present. This form of N is readily available for plant uptake, but is prone to losses as ammonia if not incorporated within 12 hours after application. Assuming direct manure incorporation after application, 45 to 75% of the total N (organic-N + ammonium-N) is available the first year. Note that for composted manure, the percentage of the organic N available in the first year following application is much lower than it is for fresh manure. Because there is very little ammonium-N in composted manure, the organic N fraction is basically the same as the total N fraction.
Bedding or litter will usually decrease nutrient content by dilution. If materials high in carbon (C) like straw or wood shavings are used as bedding, N availability may be reduced by the larger C/N ratio of the product. High C relative to N will lead to a tie-up of N, potentially causing N deficiency in the crop. A C/N ratio of 25/1 or greater will lead to N tie-up in the soil. A C/N ratio of less than 25/1 will release N to the crop. The C/N ratio is also an important consideration in the use of various composts, as well as a controlling factor in the composting process itself.
MANURE TYPE |
Organic N |
|
|
(% available) |
| Swine, fresh |
50 |
| Beef, no bedding |
35 |
| Beef, with bedding |
25 |
| Dairy, no bedding |
35 |
| Dairy, with bedding |
25 |
| Sheep, solid |
25 |
| Poultry, no litter |
50 |
| Poultry, with litter |
45 |
| Horse, with bedding |
20 |
| Composted poultry |
30 |
| Composted dairy |
14 |
Manure and Compost Application
As discussed above, some of the N in fresh manure will be lost to the atmosphere during application in the form of ammonia gas. The higher the ammonium-N fraction is in manure, the more prone it is to ammonia volatilization. Manure should be incorporated within 12 hours of application to avoid excessive ammonia losses. Unincorporated manure will supply the organic N fraction and at most 20% of the ammonium-N fraction. Incorporation of composted manure is not as critical, because the N is stabilized in organic compounds with little free ammonium present. However, in order to obtain full benefit from compost, incorporation is recommended whenever possible. Manure and compost are often high in soluble salts, so to avoid salt injury seeding operations should take place about 3 to 4 weeks after application.
Residual Nutrients in Soil from Manure and Compost Application
The residual effects of the manure and compost are important. Some benefit will be obtained in the second and third years following application. When manure and compost are used to fertilize crops, soil organic matter will increase over time and subsequent rates of application can generally be reduced because of increased nutrient cycling. Continuous use of manure or compost can lead to high levels of residual N, P, and other nutrients, which can potentially be transported to lakes and streams in runoff or leach and pollute the groundwater. Taking into account residual release of N in subsequent years should help to avoid excessive applications. General rules of thumb for N are that organic N released during the second and third cropping years after initial application will be 50% and 25%, respectively, of that mineralized during the first cropping season. Remember that some manures and composts contain high levels of P, so if organic nutrient sources are regularly applied at rates to meet crop N demands, the amount of P in the soil can build up to excessively high levels. Use of soil tests, plant tissue tests, and monitoring of crop growth will help in determining the amount of residual N and other nutrients in the soil and the need for further applications.
Calculating the Amount of Manure or Compost to Apply
Methods for calculating the amount of manure or compost to apply have been adapted and summarized from Livestock Waste Facilities Handbook, 2nd ed., 1985, Midwest Plan Service. Composts can be thought of as similar to manure, but with little or no ammonium-N present. The amount of compost required to meet crop nutrient demands can be very large. For these situations, more readily available nutrients from other sources may be required to supplement compost additions, especially early in the growing season.
Use the following steps to determine the manure or compost rate needed for a particular crop:
Step 1
- Determine the nutrient needs of the crop – Base nutrient needs on soil test recommendations.
Step 2
- Determine the total nutrient content of the manure or compost – Chemical analysis of the actual product is strongly recommended; a general estimate can be obtained from Table 1 above.
Step 3
- Determine the available nutrient content– Use 80% availability for P2O5 and 90% availability for K2O. Calculate N availability using the following equation:
| Available N = (Organic N x Km) + Ammonium-N* |
Where:
|
| *Note: if manure is not incorporated within 12 hours after application, reduce the value for ammonium-N using Table 3 to account for volatilization losses; reduce ammonium-N in the Available N equation, but use the full value in the equation for Organic N |
|
Days Until Incorporation |
% of Ammonium-N Available to Crop |
|
0.5-2 |
80 |
|
2-4 |
60 |
|
4-7 |
40 |
|
>7 |
20 |
Step 4
- Calculate the rates of application needed to supply the recommended amounts of N, P2O5, and K2O – Divide the recommended nutrient needs from Step 1 by the pounds of available nutrients per ton of manure or compost determined in Step 3.
Step 5
- Select the rate of manure or compost to apply– Frequently, manure and compost application rates are based on the N need of the crop. If manure or compost is applied on a regular basis, you may need to base rates on P to avoid excessive buildup of P in the soil, and supplement with other N sources to meet the total crop N requirement. For legumes, either P2O5 or K2O can be used as a basis for rates, depending on crop needs and soil test levels.
Step 6
- Determine the amount of available nutrients applied with the manure or compost – multiply the application rate of manure or compost determined in Step 5 (in tons/A) times the estimated available nutrients (in lb/ton) determined in Step 3. The amounts calculated can be compared with crop needs (from Step 1) to determine if supplemental nutrients are needed (next Step).
Step 7
- Determine whether application of additional nutrients is needed–Subtract the amount of nutrients needed by the crop (based on the soil test in Step 1) from the amounts of available nutrients applied with the manure or compost (calculated in Step 6). If the number obtained for a nutrient is zero or negative, then no further application is necessary. A positive number indicates the amount of that nutrient (in lb/A) that needs to be applied from another nutrient source to meet crop demands.
Example Calculation
The following steps provide an example manure rate calculation for the following situation:
Step 1 – Determine the nutrient needs of the crop
Step 2 – Determine the total nutrient content of the manure
Step 3 – Determine the available nutrient content
Available P2O5 = 0.80 x Total P2O5
Available K2O = 0.90 x Total K2O
Step 4 – Calculate the rates of application needed to supply the recommended amounts of N, P2O5, and K2O
Step 5 – Select the rate of manure to apply
Step 6 – Determine the amount of available nutrients applied with the manure
Step 7 – Determine whether application of additional nutrients is needed
|