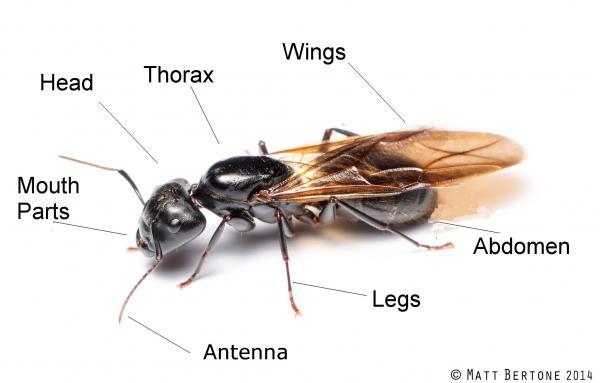
Case Study—Think about IPM: A Cantaloupe Problem
You are concerned about your cantaloupe plant. The vines are doing poorly, some of the fruit has scarring, and you are concerned you will lose the whole crop. You review the diagnostic procedures from chapter 7, “Diagnostics“, and think about the five steps for IPM:
- Monitor and scout insects to determine insect type and population levels.
- Accurately identify pest and host.
- Consider economic or aesthetic injury thresholds. A threshold is the point at which action should be taken.
- Implement a treatment strategy using physical, cultural, biological, and/or insecticide control.
- Evaluate success of treatments.
1. Monitor and determine insect type
You have noticed the plants not doing well for a few weeks. An insect that looks like a beetle has been flying around the crop. The plants have been in the ground for a few months (are mature) and they appear to have insect damage to their leaves. A younger planting of the same crop suffered from severe damage, and the stems appeared chewed through. Cantaloupe has been planted in the same spot for many years. You feel the insect population is high enough and damage is severe enough to warrant further investigation. You take a sample of the leaves getting leaves that are healthy, partially affected and completely affected by damage. You also try to capture the insect if possible or at least get some good photographs. You review the sample collection information found in the “Submitting Insect Samples to the Plant Disease and Insect Clinic” section of this chapter.
2. Accurately identify the pest and host
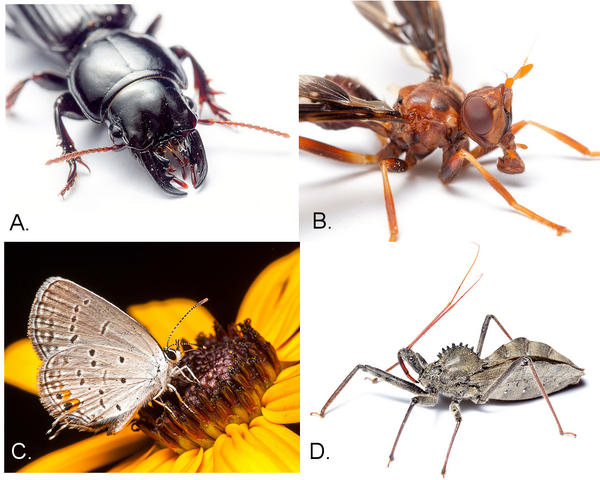
Using the samples you research this problem. The leaves appear to be missing sections, especially along the margins, which leads you to believe an insect with chewing mouthparts was present. You carefully examine the sample to see if any insects are still present. You see a yellow and black insect and remove it from the plant for further investigation. The insect is quite active, so you put it in a jar and freeze it to slow it down. Looking at it with your hand lens, you determine it is in the order Coleoptera, or a beetle, because you see two pairs of wings; the outer pair is hardened or leathery with yellow and black stripes, and the inner pair is membranous and folds under the front wings. You also see chewing mouthparts and long jointed antennae. You consult some insect identification guides at the Extension office and discover a photo that looks like your insect (Figure 4–40). You read that cucumber beetles can attack all plants in the cucurbit family and that cantaloupe is in that family. Now that you have a positive ID, you do more research on this insect. You type in “cucumber beetle + extension” into your Internet search engine.
3. Consider economic or aesthetic injury thresholds
The injury to these vines and their fruit is severe enough to warrant management. It may be too late for this year’s crop, but you can research how best to avoid this problem in future plantings.
4. Implement a treatment strategy using physical, cultural, biological, and/or insecticide control
You research cultural strategies to discourage cucumber beetles. Cucumber beetle eggs often overwinter in discarded plant material, so removal of plant material after the growing season and plowing or turning the planting bed will help disrupt this part of the beetle’s life cycle. Crop rotation can be an effective means of managing soil fertility and pest problems. Ideally, plant a different crop family in the spot, waiting at least three years before returning to a cucurbit family crop. The use of resistant varieties is perhaps the most important management tactic. The following cucurbit varieties are resistant to cucumber beetles as seedlings and also have resistant foliage later in the season: Blue Hubbard (squash); Ashley, Chipper, Gemini (cucumber). Use of resistant varieties may not give complete control where infestations are heavy. The NC Cooperative Extension publication AG-295, Insect and Related Pests of Vegetables, gives additional cultural management strategies.
Delaying planting times for more favorable germinating conditions and heavy seeding rates will ensure a good stand. For young seedlings, wire or cloth screen protectors shaped like cones will keep beetles off home plantings until plants are established.
If this combination of management strategies is not enough, insecticides may be needed. In that case, a foliar insecticide applied at the cotyledon stage will retard cucumber beetle feeding and encourage plant establishment. Where insects are abundant, additional foliar applications may be needed to prevent beetles from spreading bacterial wilt and squash virus. For recommended insecticides and rates, consult the current North Carolina Agricultural Chemicals Manual.
5. Evaluate success of treatments
You decide to keep a garden journal or notes about management strategies tried and their results. You realize that it may take more time to see the results of some strategies than others.

Figure 4-40. A striped cucumber beetle, Coleoptera order.
Matt Bertone
Further Reading
Bellows, Thomas S. and T W. Fisher, eds. Handbook of Biological Control: Principles and Applications of Biological Control. San Diego, California: Academic Press, 1999. Print.
Brandenburg, Rick L. and Callie P. Freeman, eds. Handbook of Turfgrass Insects, Second Edition. Annapolis, Maryland: Entomological Society of America, 2012. Print.
Bruneau, Arthur H. and Gail G. Wilkerson, eds. Turfgrass Pest Management Manual: A Guide to Major Turfgrass Pests & Turfgrasses. Raleigh, North Carolina: North Carolina Cooperative Extension Service, 2006. PDF file.
Capinera, John L. Handbook of Vegetable Pests. San Diego, California: Academic Press, 2001. Kindle file.
Clark, Christopher A., et al., eds. Compendium of Sweetpotato Diseases, Pests, and Disorders. 2nd ed. St. Paul, Minnesota: The American Phytopathological Society, 2013. Print.
Common Tree Fruit Pests. Columbia, Missouri: University Of Missouri Extension, 1993. Print. North Central Regional Publication NCR 63
Cottam, Clarence, and Herbert S. Zim. A Golden Guide from St. Martin’s Press: Insects. New York: St. Martin’s Press, 2002. Print.
Cranshaw, Whitney. Garden Insects of North America. Princeton, New Jersey: Princeton University Press, 2004. Print.
Davidson, Ralph H., and William F. Lyon. Insect Pests of Farm, Garden, and Orchard. 8th ed. Hoboken, New Jersey: John Wiley & Sons Inc., 1987. Print.
Dreistadt, Steve H. Pests of Landscape Trees and Shrubs: An Integrated Pest Management Guide. 2nd ed. Davis, California: University of California Division of Agriculture And Natural Resources, 2004. Print. Publication 3359.
Ellis, Barbara W. and Fern Marshall Bradley, eds. The Organic Gardener’s Handbook of Natural Insect and Disease Control: A Complete Problem-Solving Guide to Keeping Your Garden and Yard Healthy without Chemicals. Emmaus, Pennsylvania: Rodale Press, Inc., 1996. Print.
Ellis, M. A., et al., eds. Compendium of Raspberry and Blackberry Diseases and Insects. St. Paul, Minnesota: The American Phytopathological Society, 1991. Print.
Elzinga, Richard J. Fundamentals of Entomology. 6th ed. Upper Saddle River, New Jersey: Prentice Hall, Inc., 2004. Print.
Horst, R. Kenneth, and Raymond Cloyd. Compendium of Rose Diseases and Pests. 2nd ed. St. Paul, Minnesota: The American Phytopathological Society, 2007. Print.
Johnson, Warren T., and Howard H. Lyon. Insects That Feed on Trees and Shrubs. 2nd ed. Ithaca, New York: Cornell University Press, 1991. Print.
Jones, Jeffrey B., et al., eds. Compendium of Tomato Diseases and Pests. 2nd ed. St. Paul, Minnesota: The American Phytopathological Society, 2014. Print.
Leahy, Christopher. Peterson First Guide to Insects of North America. New York: Houghton Mifflin Company, 1998. Print.
Mitchell, Robert T., and Herbert S. Zim. Butterflies and Moths: Revised and Updated. New York: St. Martin’s Press, 2002. Print.
Pirone, Pascal P. Diseases and Pests of Ornamental Plants. 5th ed. New York: John Wiley & Sons, Inc., 1978. Print. Note: This is a good resource for diagnostics; refer to the North Carolina Agricultural Chemicals Manual for the latest chemical information.
Schultz, Warren, ed. Natural Insect Control: The Ecological Gardener’s Guide to Foiling Pests. 1995. Brooklyn, New York: Brooklyn Botanic Garden, 1999. Print.
Schwartz, Howard F. and S. Krishna Mohan, eds. Compendium of Onion and Garlic Diseases and Pests, Second Edition. St. Paul, Minnesota: The American Phytopathological Society, 2008. Print.
Solomon, J. D. Guide to Insect Borers in North American Broadleaf Trees and Shrubs. Washington, DC: United States Department of Agriculture Forest Service, 1995. PDF file. Agriculture Handbook AH-706.
Sutton, Turner B., et al., eds. Compendium of Apple and Pear Diseases and Pests. 2nd ed. St. Paul, Minnesota: The American Phytopathological Society, 2013. Print.
Weinzierl, Rick, and Tess Henn. Alternatives in Insect Management: Biological and Biorational Approaches. Urbana, Illinois: University of Illinois At Urbana-Champaign, 1991. PDF file. North Central Regional Extension Publication 401.
Weinzierl, Rick, and Tess Henn. Alternatives in Insect Management: Microbial Insecticides. Urbana, Illinois: University of Illinois At Urbana-Champaign, 1989. PDF file. Circular 1295.
Weinzierl, R., et al. Insect Attractants and Traps. Gainesville, Florida: University of Florida Institute of Food And Agricultural Science, 2005. PDF file. Publication ENY-277.
Yepsen, Roger B, ed. The Encyclopedia of Natural Insect and Disease Control: The Most Comprehensive Guide to Protecting Plants, Vegetables, Fruit, Flowers, Trees and Lawn. Emmaus, Pennsylvania: Rodale Press, Inc., 1984. Print.
 DESCRIPTION
DESCRIPTION