Phphosphorus_deficiency1osphorus is a vital component of adenosine triphosphate (ATP), the ‘energy unit’ of plants. ATP forms during photosynthesis, has P in its structure, and processes from the beginning of seedling growth through to the formation of grain and maturity.
Plumeria Care
Periodic Table of Crop Nutrients and Deficiencies
Related Images:
The Role of Nitrogen (N)
Nitrogen (N) is essential for plant growth and is part of every living cell. It plays many roles in plants and is necessary for chlorophyll synthesis. Plants take up most of their N as the ammonium (NH4+) or nitrate (No3-) ion. Some direct absorption of urea can occur through the leaves, and small amounts of N are obtained from materials such as water-soluble amino acids.
Plumeria – Frangipani mosaic virus
 196 August 1978 | Family: Virgaviridae Genus: Tobamovirus Species: Frangipani mosaic virus Acronym: FrMV |
Frangipani mosaic virus
A. Varma – Division of Mycology and Plant Pathology, Indian Agricultural Research Institute, New Delhi 110012, India
A. J. Gibbs – Research School of Biological Sciences, Australian National University, Canberra, Australia
Contents
| Introduction Main Diseases Geographical Distribution Host Range and Symptomatology Strains Transmission by Vectors Transmission through Seed Transmission by Grafting Transmission by Dodder Serology Nucleic Acid Hybridization Relationships Stability in Sap | Purification Properties of Particles Particle Structure Particle Composition Properties of Infective Nucleic Acid Molecular Structure Genome Properties Satellites Relations with Cells and Tissues Ecology and Control Notes References Acknowledgements Figures |
Introduction
- Described by Francki, Zaitlin & Grivell (1971).
- Synonym
- Temple tree mosaic virus. A virus with tubular particles 300 nm long and 18 nm in diameter. Sap transmissible. No vector known; is spread in cuttings of infected frangipani (Plumeria spp.). Restricted host range, grows best at 30-35°C.
Main Diseases
In Plumeria acutifolia the virus causes mosaic, ringspots, veinbanding and bronzing. In P. alba, it causes ringspots, leaf distortion and necrosis. No flower symptoms.
Geographical Distribution
Common in eastern Australia and northern India.
Host Range and Symptomatology
Host range not yet tested extensively, but seems restricted. More species become infected at temperatures above 25°C than below. At 35°C symptoms show in 3-6 days, at 22°C they take 2 weeks or more.
- Diagnostic species
- Datura stramonium. Chlorotic, necrotic or black lesions develop in inoculated leaves after 1-2 weeks in the glasshouse at 22°C. At 35°C, in controlled environment cabinets, similar symptoms develop in 3 days; one strain causes systemic necrosis along the veins and leaf margins.
- Nicotiana glutinosa. At 22°C inoculated leaves develop chlorotic lesions in about 2 weeks. Not infected systemically.
- N. tabacum (tobacco) cvs Samsun, Virginia Gold or White Burley. Rarely infected at 22°C. At 35°C all strains induce bright chlorotic or necrotic ringspots in inoculated and systemically infected leaves.
- N. clevelandii x N. glutinosa. Not infected at 22°C. At 35°C inoculated leaves develop faint chlorotic lesions which become necrotic or develop ringspots. Not infected systemically.
- Propagation species
- Nicotiana glutinosa. Inoculated leaves give a good yield after 2-3 weeks at 22°C.
- Assay species
- Datura stramonium is the most reliable assay species.
Strains
Three distinct strains from different provenances have been distinguished by the symptoms they produce. They are the Adelaide strain (Adel) (Francki et al., 1971), and the Allahabad (Ald) and Delhi (Del) strains (A. Varma & A. J. Gibbs, unpublished data). Leaves of D. stramonium kept at about 22°C develop faint chlorotic lesions after inoculation with strain Adel, necrotic lesions after inoculation with strain Ald, and chlorotic lesions, later becoming black, after inoculation with strain Del. At 35°C symptoms developed more quickly and spread more: strain Adel gave necrotic lesions, strain Ald gave lesions with chlorotic haloes or ringspots, and strain Del gave spreading black necrotic ringspots and systemic veinal and marginal necrosis. N. tabacum cv. Virginia Gold was susceptible at 22°C to strain Del only, showing chlorotic and necrotic local lesions. At 35°C in the same tobacco cultivar, strain Adel gave faint necrotic ringspots, strain Ald gave bright necrotic ringspots and strain Del gave large ringspots both in inoculated and in tip leaves.
Transmission by Vectors
No vector is known.
Transmission through Seed
Not transmitted through seed of D. stramonium or N. tabacum cv. Samsun.
Serology
Particles of the virus are strongly immunogenic. They give flocculent precipitates in tube precipitin tests, and form one band of precipitate in gel diffusion tests.
Relationships
Properties, serological relationships and particle morphology place the virus in the tobamovirus group. The particles of frangipani mosaic virus are morphologically indistinguishable from those of other tobamoviruses. The Adel, Ald and Del strains are serologically closely related to each other. All three strains are related distantly to cucumber virus 4, cucumber green mottle mosaic virus, and an isolate of sunn-hemp mosaic virus from Queensland, Australia (but not one from West Africa); and even more distantly to TMV-type strain, TMV-U2 strain, tomato mosaic virus and ribgrass mosaic virus. (A. J. Gibbs & A. Varma, unpublished data; Franckiet al., 1971). There was no detectable serological relationship with Sammons’ opuntia virus even though comparisons of coat protein composition indicate a close affinity (Description No. 184).
Stability in Sap
Very stable. Sap from infected D. stramonium was not infective after heating to 95°C for 10 min, and lost 90% of its infectivity in 10 min at 90°C. The sap was still infective after 10 weeks at room temperature, and at dilutions up to 10-5.
Purification
The virus is easily purified from infected leaves of frangipani or N. glutinosa by several methods. The following methods give good yields:
1. Francki et al. (1971), based on McLean & Francki (1967) and Francki & McLean (1968). Homogenise infected leaves of N. glutinosa in 1.5 volumes of 0.2 M Na2HPO4, clarify by adsorption with charcoal and DEAE cellulose and filter through Celite. Sediment the particles by centrifuging at 44,000 g for 90 min. Resuspend pellets in distilled water and emulsify with equal volume of chloroform. Centrifuge at 12,000 g for 10 min. Collect aqueous layer and sediment the particles by centrifuging at 16,000 g for 30 min. Repeat chloroform extraction and sedimentation.
2. Based on Varma, Gibbs & Woods (1970). Triturate infected leaves mechanically with 2 ml/g of neutral phosphate-ascorbate buffer (equal volumes of 0.1 M disodium hydrogen phosphate and 0.05 M ascorbic acid). Add a quarter volume of chloroform, emulsify, centrifuge at 8000 g for 10 min, collect supernatant fluid and centrifuge for 1 h at 75,000 g. Resuspend the pellets in a small quantity of the buffer. Further purify by rate zonal centrifugation at 45,000 g for 75 min in gradients of 10-40% sucrose.
Properties of Particles
In dilute solutions the virus sediments as a single component with sedimentation coefficient (s20, w) of 188 S (R. D. Woods, unpublished data).A260/A280: 1.21.
Particle Structure
The virus has rod-shaped particles about 300 nm long and 17 nm wide. The preparations also contain shorter particles (Francki et al., 1971) (Fig.6).
Particle Composition
Nucleic acid: The particles contain c. 5% RNA.Protein: Each subunit of the coat protein of strain Adel contains about 158 amino acid residues: Ala, 14; Arg, 11; Asx, 17; Cys, 1; Glx, 16; Gly, 9; His, 1; Ile, 11; Leu, 13; Lys, 4; Met, 0; Phe, 7; Pro, 4; Ser, 14; Thr, 13; Trp, 5; Tyr, 5; Val, 13 (Francki et al., 1971). Of the other tobamoviruses whose coat proteins have been analysed, Sammons’ opuntia virus has a composition most similar to that of frangipani mosaic virus..
Relations with Cells and Tissues
In the cytoplasm of infected parenchymatous cells of D. stramonium leaves, the particles of frangipani mosaic virus aggregate as microcrystals of various shapes and sizes. Particles were not seen in mitochondria, chloroplasts or nuclei although these organelles are not of normal appearance.
References
- Francki & McLean, Aust. J. biol. Sci. 21: 1311, 1968.
- Francki, Zaitlin & Grivell, Aust. J. biol Sci. 24: 815, 1971.
- McLean & Francki, Virology 31: 585, 1967.
- Varma, Gibbs & Woods, J. gen. Virol. 8: 21, 1970.
Related Images:
Over Watering your Plumeria
Related Images:
How to read a Fertilizer Label
Related Images:
Branching Inducers on Plumeria
Branching Inducers on Plumeria
To produce more branches per cut from a pruned plumeria limb. UH experiments suggest that lanolin pastes of cytokinins can improve the number of buds which break following pruning. It is desirable to increase lateral shoot production after pruning for cultural control both in commercial flower production and landscapes as well as for plants grown in containers (Kwon and Criley, 1991).
Procedure:
Apply 6-benzylaminopurine (BA) in a lanolin paste to pruned tips. Kwon and Criley (1991) used single stemmed 2 year old plants of P. rubra in 35 x 22 cm pots containing a medium of equal parts soil, peat, and perlite. The plants were decapitated at 30 cm above the soil line 3 weeks after repotting. Various concentrations of the growth regulator solution was then applied to be absorbed into the cut surface. We will use 2 mg/g and 4 mg/g (BA/lanolin) solutions (2000 and 4000ppm). Four plants should be used for each treatment plus 4 control plants. Data to collect are; (a)number of days to bud break for each plant, (b)number of shoots initiated and (c)surviving and (d)the length of these at 4 months after treatment.
The following passage is quoted from a letter from Dr. Richard A. Criley, dated September 16, 1994. It outlines where BA and lanolin may be obtained, how to mix them, how to apply the paste to plumeria, and addresses the question of the shelf life of the mixture.
“The application of chemical branch inducing substances in lanolin would be an interesting set of studies for PSA members to try. You can get plain old lanolin at many pharmacies or order it through chemical supply houses. It comes as a yellow, very sticky fatty substance. You can melt it in a double boiler and dissolve or suspend substances in it. My recommendation is to weigh out the stuff in the same container you want to melt it in – then you are sure of concentrations.
The benzyladenine is not water soluble to any great extent. I usually dissolve it in a small amount of acetone or alcohol or DMSO. You can also use 0.5 N HCL (small amount) to dissolve it, then dilute in water and add to the melted lanolin. Stir well to make a uniform mixture. Allow to cool. I use it by smearing a glob on the cut stump of a plumeria branch using a finger covered with a latex glove finger. Rates to try: 2 or 4 mg BA in 1 gram of lanolin. I have a mixture from 4 – 5 years ago that still has activity.
You can also pour the BA-lanolin paste mix while still liquid into plastic film canisters. This might be one way to share it around easily. (Maybe you could charge a few dollars per unit and recover the costs.)
The N-6-benzyladenine (BA) is also known as 6-benzylaminopurine.”
Summary:
- Use 6-benzylaminopurine (BA) in a lanolin paste.
- Apply paste to pruned tips. (1) 2 mg BA per gram lanolin. (2) 4 mg BA per gram lanolin.
- These experiments would need control plants which receive a no N-6-benzyladenine . Both the experimental subject and control plant would have to be of the same cultivar to avoid inconsistencies in growth habit and flowering.
References:
Eunoh Kwon and Richard A. Criley (1991), Cytokinin and Ethephon Induce Greater Branching of Pruned Plumeria, Horticulture Digest; Hawaii Cooperative Extension Service, No. 93, March 1991, p. 6-8.
6-benzylaminopurine from Sigma Chemical Company; catalog # B 3408: 1 g, $10.65 + shipping. Lanolin from Sigma Chemical Company; catalog # L 7387: 1 kg, $32.80 + shipping.
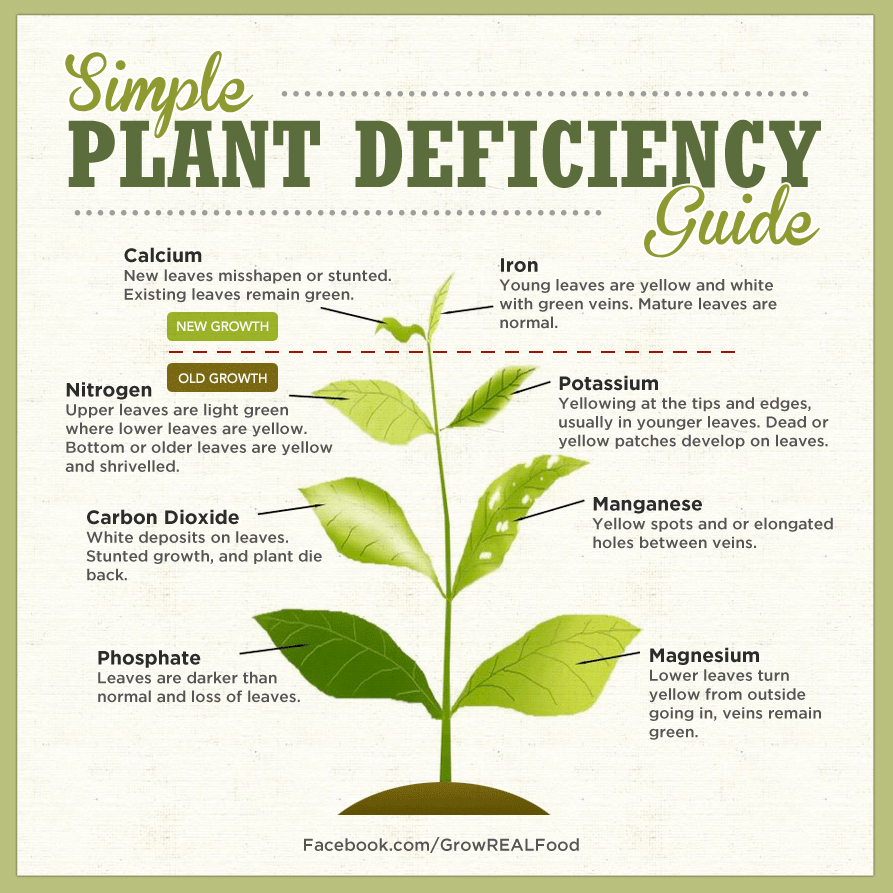

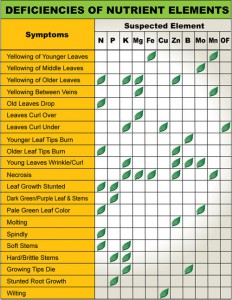 A good reference to get an idea if your Plumeria are Deficient in Nutrients
A good reference to get an idea if your Plumeria are Deficient in Nutrients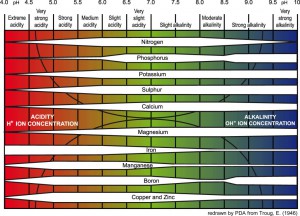 If your PH is too high or too low you nutrients could be locked in the soil.
If your PH is too high or too low you nutrients could be locked in the soil. Phosphorus (K) deficiency guide
Phosphorus (K) deficiency guide



